Sugarcane smut
Pacific Pests, Pathogens and Weeds - Online edition
Pacific Pests, Pathogens & Weeds
Sugarcane smut (474)
Sporisorium scitamineum; previously known as Ustilago sacchari, and Ustilago scitaminea. Genetic diversity appears to be greatest in Asia, but whether there are strains of the fugus is more controversial.
Africa, Asia, North, South and Central America, the Caribbean, Europe, Oceania. It is recorded from Australia. The CABI Crop Protection Compendium notes against the record for Fiji: "Absent: Invalid presence records(s)". It was previously recorded from Fiji as Ustilago sacchari (Dingley JM, et al. (1981).
Sugarcane, and related Saccharum species, edible and wild. While some reports mention grass species, others suggest that only Saccharum species are hosts.
A serious fungal disease of sugarcane. A characteristic of the disease is the production of spore-bearing, black, whip-like structures from the growing point (Photos 1&2). Severely infected plants may be stunted with small, narrow leaves, and abundant tillers; they look weak and grass-like. More than one flush of whips can cause considerable damage to crops of susceptible varieties.
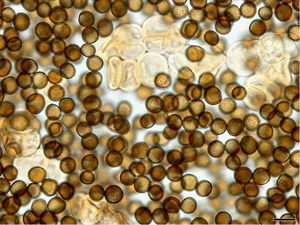
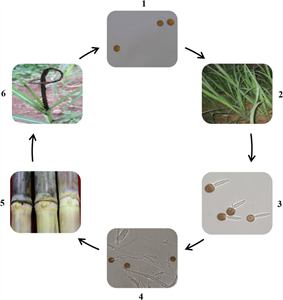
Buds either above or below ground become infected by spores (called teliospores) of the fungus. The spores germinate in water producing two types of mating spores (called sporidia); these combine to produce fungal growth which penetrates the bud. The fungus remains in the buds until cuttings are prepared, planted, and the buds begin to grow. As the buds grow, so does the fungus, and in the process it colonises the shoot. Eventually, the leaf spindle - young leaves and growing point - enclosed within the sheaths of older leaves is replaced with a long black whip, which emerges from the top of the plant. Smaller whips may develop from lateral buds giving rise to grass-like stems. At first, the sooty black-brown, roughly round spores that develop in the whip are covered by a thin membrane, but this breaks eventually to release them (Photo 3). The life-cycle is shown (Photo 4).
Spread occurs via spores blown on the wind, and from the use of infected cuttings for planting. Other possibilities are spread of spores on machinery and footwear. Survival occurs in soil for 3 to 4 months and in infected plants as long as they remains alive.
Production losses of 30 to 100% are reported depending on the susceptibility of the variety, the strain of the fungus, the environment (the disease is worse in hot dry climates) and the age of the crop when infected. There is also a loss of quality as stems may have lower sugar content. Breeding programs may be impacted because of the susceptibility of valuable lines. An approximate estimate of yield loss (in Australia) is 0.6% for every 1% increase in the number of infected plants. In the case of susceptible varieties, large numbers of dead plants can result in the loss of the ratoon crop, and additional costs due to replanting.
CABI reports that the disease appears to be cyclical, although it is not known why this is so. Epidemics of the disease are often followed by periods when the disease is of minor importance.
Look for stunted plants and the characteristic black whips that emerge from the top of the stems. ELISA and PCR-based methods are available for detection of the fungus in buds of sugarcane, and a reliable staining method using trypan blue has also given reliable results.
BIOSECURITY
Because diseases of sugarcane are unevenly distributed throughout the world, all efforts should be made to prevent their further spread. Sugarcane plants moved internationally should follow the FAO/IBPGR Technical Guidelines for the Safe Movement of Sugarcane Germplasm. Frison EA, Putter CAJ (eds.). 1993. Rome, Italy: (http://www.bioversityinternational.org/uploads/tx_news/Musa_spp.__2nd_edition__502.pdf).
CULTURAL CONTROL
Before planting
- Deep plough or irrigate fields following outbreaks to reduce the level of viable spores in the soil or in sugarcane debris. Teliospores lack dormancy, lasting only 2-3 months.
- Use hot water treatment to eliminate infections. Cuttings are immersed in water at 52oC for 30 minutes. Alternatively, 50oC for 3 hours. A method used widely for relatively small planting of sugarcane as well as large plantations. Note, however, that breeding for resistance remains the most important method for control of sugarcane smut.
During growth
- In small plantations, and at relatively low levels of infection, hot water treatment should be used in combination with removal of infected plants. Rogueing is done principally to lower infection of stems, in order to protect cuttings of the next crop.
RESISTANT VARIETIES
The production of varieties with resistance is the main method used to manage sugarcane smut. However, the level of resistance varies between locations depending on environment as dry, warm conditions favour the disease. Resistance appears to be related to the ability of spores to infect buds and/or the growth of the fungus within buds.
CHEMICAL CONTROL
This is not an appropriate method to use for controlling this disease, apart from the use of fungicides after hot water treatment. Usually, cuttings are dipped in fungicide (flutriafol or propiconazole) after hot water treatment to protect the buds, which may become soft and more easily infected.
____________________
When using a pesticide, always wear protective clothing and follow the instructions on the product label, such as dosage, timing of application, and pre-harvest interval. Recommendations will vary with the crop and system of cultivation. Expert advice on the most appropriate pesticides to use should always be sought from local agricultural authorities.
AUTHOR Grahame Jackson
Information from Sugar Research Australia. Sugarcane smut. Information sheet ISI3012. (https://sugarresearch.com.au/wp-content/uploads/2017/02/Sugarcane-smut-IS13012.pdf); and CABI (2019) Sporisorium scitamineum (sugarcane smut) Crop Protection Compendium. (https://www.cabi.org/cpc/datasheet/55949); and Sugarcane smut. Wikipedia. (https://en.wikipedia.org/wiki/Sugarcane_smut); and from Dingley JM, et al. (1981) Records of fungi, bacteria, algae, and angiosperms pathogenic on plants in Cook Islands, Fiji, Kiribati, Niue, Tonga, and Western Samoa. Technical Report (2). South Pacific Bureau for Economic Co-operation, United Nations Development Programme, Food and Agricultural Organisation of the United Nations. Photo 1 William M. Brown Jr.Bugwood.org. Photos 2&3 Roger Sivas (2010) Sugarcane smut (Ustilago scitaminae: PaDIL - (http://www.padil.gov.au). Photo 4 Que Y, et al. (2014) Genome sequencing of Sporisorium scitamineum provides insights into the pathogenic mechanisms of sugarcane smut. BMC Genomics 2014(15):996. (https://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-15-996).
Produced with support from the Australian Centre for International Agricultural Research under project HORT/2016/185: Responding to emerging pest and disease threats to horticulture in the Pacific islands, implemented by the University of Queensland and the Secretariat of the Pacific Community.