Sweetpotato virus disease.
Pacific Pests, Pathogens, Weeds & Pesticides - Online edition
Pacific Pests, Pathogens, Weeds & Pesticides
Sweetpotato virus disease (528)
Sweet potato chlorotic stunt virus and Sweet potato feathery mottle virus interact to cause sweet potato virus disease. There are different strains of both viruses in East Africa, and differences between the strains there and those elsewhere in Africa. The abbreviation of the disease is SPVD, and for the two viruses the abbreviations are SPCSV (see Fact Sheet no. 375) and SPFMV (see Fact Sheet no. 258), respectively. SPCSV belongs to the genus Crinivirus; SPFMV belongs to the genus Potyvirus.
Sweetpotato virus disease occurs throughout sub-Saharan Africa; it is particularly common in the Great Lakes region. Both SPCSV and SPFMV occur worldwide in all the sweet potato regions of Asia, Africa, North and South America and Oceania.
Sweetpotato.
Sweet potato virus disease was first reported in the 1940s, it was not until 30 years later that the cause was known. The two viruses involved do relatively little damage on their own (see Fact Sheet nos. 375, 258).
Severe SPVD symptoms occur because the presence of SPCSV turns offf the resistance mechanism which normally keeps SPFMV in check, allowing SPFMV to reach much higher concentrations (up to 600 times) than if it were alone. Symptoms may become even worse if a third virus infects the plants: there are over 30 different types of virus that infect sweet potato.
When the two viruses occur together in susceptible sweet potatoes, symptoms can be highly variable with infected plants showing yellow blotches, mosaics and mottles, on leaves which are often deformed, and vines which are stunted (Photos 1&2). Yields of storage roots are generally low, but it depends when infection occurs; if it is early, storage roots fail to develop.
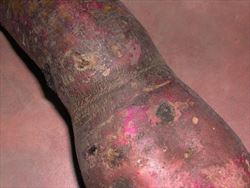
By contrast, infection by SPCSV alone results in a mild yellowing or reddening of older leaves and stunting, as its name suggests. Infection by SPFMV alone results in yellow spots or purple ringspots and, occasionally, feather-like patterns bordering the major leaf veins. On the storage roots, some strains, on certain varieties, cause networks of small cracks in the skin, or longitudinal fissures, in circular bands one or more centimetres wide (Photos 3&4). Inside, the storage roots may show black spots. These symptoms are known as russet crack and internal cork, respectively.
Often, neither virus produces symptoms in sweet potato when alone, or the symptoms occur only when plants are under stress and growing slowly; for instance, when there is not enough rain. If growing conditions improve, the rapidly growing vines often appear healthy.
Spread of SPCSV and SPFMV occurs in three ways.
First, they are spread between plants by insects: SPCSV by the whitefly, Bemisia tabaci, and SPFMV by aphids, e.g. Aphis gossypii, Aphis craccivora, and Myzus persicae. The viruses are picked up as the insects feed on plant sap. Once aphids have the virus on their mouthparts, they can then infect healthy plants immediately, but this ability to infect is lost quickly. By contrast, whiteflies take a few hours before they are ready to spread SPCSV, but they can continue to infect for longer.
Secondly, the viruses are spread in cuttings used for planting.
Thirdly, the viruses are spread in storage roots sent to markets; farmers often buy the roots and grow sprouts from them for planting; this is a way of introducing a new variety and a new type of virus.
Survival of the viruses between crops or cropping seasons occurs in vines left in the field after harvest, in storage roots discarded in the field or intentionally kept as a source of planting material, or in wild Ipomoea species such as morning glory.
SPVD is the most serious disease of sweet potato in Africa and perhaps the world. It is particularly severe in East Africa, with losses of 50-90% in the yield of susceptible plants. However, because of the disease, farmers now mostly plant tolerant varieties, and in most districts of Uganda and Kenya virus symptoms only occur in 10-20% of plants, with about a fifth to a third of those infected with both SPFMV and SPCSV. Therefore, the real impact of the disease may be an opportunity cost: farmers are forced to grow low-yielding varieties that are resistant to SPVD. However, with improved NASPOT varieties, this is becoming less of a problem.
Look for stunted plants with yellow and green mosaic patterns and mottles on the leaves. Both components of the disease can be detected and identified by ISEM, and/or ELISA with monoclonal or polyclonal antisera, or by PCR (see Fact Sheets nos. 375, 258).
BIOSECURITY
Many countries in Asia, South America and Oceania are yet free from sweetpotato virus disease, even though the two viruses that cause it are present. This may indicate that different strains are present in Africa and that precautions should be taken to avoid their introduction. The most likely pathway of entry is associated with unofficial transfers. For those that are officially sanctioned should follow the advice given in the FAO/IBPGR Technical Guidelines for the Safe Movement of Sweet Potato Germplasm (http://www.bioversityinternational.org/e-library/publications/detail/sweet-potato/).
CULTURAL CONTROL
Before planting:
-
Encourage farmers to consider the choice of planting material carefully:
-
Use varieties with resistance (see below). Farmers should be encouraged to use these varieties, not only for their tolerance to disease, but also because growing large areas will reduce infection of susceptible varieties if grown nearby.
- If unsure that cuttings are disease free, grow cuttings in nurseries for later planting in the field; this avoids farmers taking cuttings from crops that may have unseen SPVD infections and cuttings that may develop the disease when planted. Infected plants should be checked in the nursery and any with SDVD discarded.
-
- Plant new plots at least 15 m from older crops where diseased plants might be present; this gap may limit whiteflies with SPCV from invading new crops. It is better if this gap is filled with a barrier crop, especially if the barrier is tall, e.g., maize.
-
Do not plant new plots download from older plots; this is to prevent aphids and whiteflies from carrying the viruses on the wind to new crops.
-
Remove plants with SPVD, especially during the first month or so after planting; this practice can benefit the use of tolerant and also susceptible varieties. The reason for this is complex: SPCSV allows SPSMV to multiply many time more than if present alone, so preventing its spread is important. Whiteflies, spread SPCSV, but they move only short distances - about 15 m - from their host plants. Therefore, early removal of infected (source) plants can have a large impact.
After harvest:
- Collect vines and burn, bury or compost them. Do not allow discarded storage roots to sprout; collect and feed to livestock or bury them.
- Also, do not grow another sweet potato crop in that same field; there may be small storage roots left unharvested which may sprout and become sources of SPVD infection.
RESISTANT VARIETIES
In Uganda, where the disease is particularly severe, local varieties occur that are resistant to SPVD. These include New Kawogo, Nderera and Munyeera. They rarely get infected by the disease, although yields are poor in comparison to the potential yields of susceptible varieties. New Kawogo is particularly popular because of its tolerance to SPVD. Varieties with moderate field resistance from the Namulonge breeding programme are NASPOT 1 to 6, released in 1999, and NASPOT 11, released in 2010, with improved resistance to SPVD. Two more, NASPOT 12 and 13, were released in 2013 with field resistance to sweet potato virus disease and Alternaria bataticola (see Fact Sheet no. 527). Check to see if these varieties are available locally.
CHEMICAL CONTROL
Chemical control is not an appropriate method of managing this disease
AUTHOR Grahame Jackson
Information from Gibson RW, et al. (1998) Identification of the East African strain of sweet potato chlorotic stunt virus as a major component of sweet potato virus disease in Southern Africa. Plant Disease, 82(9): 1063; and Gibson RW, et al. (1998) Symptoms, aetiology and serological analysis of sweet potato virus disease in Uganda. Plant Pathology, 47(1): 95-102; and Clark CA, et al. (2012) Sweet potato viruses: 15 years of progress on understanding and managing complex diseases. Plant Disease 96(2):168-185. (http://apsjournals.apsnet.org/doi/pdfplus/10.1094/PDIS-07-11-0550); and Internal cork disease of sweet potato (Sweet potato feathery mottle virus). Plantwise Knowledge Bank. (http://www.plantwise.org/KnowledgeBank/Datasheet.aspx?dsid=50963); and O’Sullivan, et al. (Undated) Sweet potato virus disease. Sweet potato DiagNotes: A diagnostic key and information tool for sweet potato problems. (http://keys.lucidcentral.org/keys/sweetpotato/key/Sweetpotato%20Diagnotes/Media/Html/FrontPage/Index.htm); and Sweet potato virus disease. CABI Crop Protection Compendium. (http://www.cabi.org.ezproxy.library.uq.edu.au/cpc/datasheetreport?dsid=18603); and from Moyer JW, et al. (1989) FAO/IBPGR Technical Guidelines for the Safe Movement of Sweet Potato Germplasm. Food and Agriculture Organization of the United Nations, Rome/International Board of Plant Genetic Resources. Photos 1&2 Richard Gibson, Plant Psthologist, Natural Resources Institute, University of Greenwish, UK. Photo 4 Gerald Holmes, Strawberrry Center, Cal Poly San Luis Obispo, Bugwood.org.
Produced with support from the Australian Centre for International Agricultural Research under project HORT/2016/185: Responding to emerging pest and disease threats to horticulture in the Pacific islands, implemented by the University of Queensland, in association with the Pacific Community.