Eggplant phomopsis blight
Pacific Pests, Pathogens, Weeds & Pesticides - Online edition
Pacific Pests, Pathogens, Weeds & Pesticides
Eggplant stem canker (545)
Phomopsis vexans; the sexual state is Diaporthe. Note, the species causing eggplant blight in Pacific island countries awaits confirmation.
Asia, Africa, North, South and Central America, the Caribbean, Oceania. It is recorded in Australia, Fiji, French Polynesia, New Caledonia.
Eggplant; there are reports of other hosts in the Solanum family, including wild species, but they are unconfirmed.
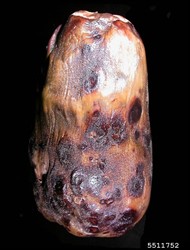
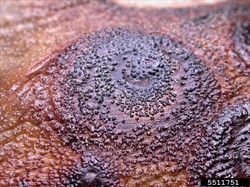
A fungus causes the damage. On fruits, oval or round, grey or brown, sunken spots occur, often with lighter central areas, developing characteristic target-like circles of small black (pinhead-sized) dots, i.e., the fruiting bodies containing the spores (Photos 1-3). Spots also occur on the leaves. On stems, dark brown areas of decay occur near soil level. As the rots advance stems may split, leaves wilt, and eventually the plants collapse and die (Photo 4&5).
Spread of Phomopsis vexans is on or in seed, or from spores in the soil. In either case, rain splash moves spores from the soil onto stems and fruit. Spread between plants is by wind-driven rain or irrigation water. Apart from seed, long-distance spread can occur from infected fruit traded internationally. In the field, the disease is favoured by hot humid conditions, with temperatures of 28-32oC.
Note, seed infection can result in poor germination or damping off (see Fact Sheet no. 47).
As a result of fruit infections, loses of 20-50% have been reported from many countries. Further, seed infection can lead to both pre-emergence and post-emergence damping off. In Bangladesh, this has been estimated to be up to 80% in nurseries.
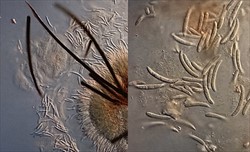
Look for basal stem lesions; look for fruits with large lesions with black concentric rings, against a grey, brownish background. But note, the appearance of the lesions is very similar to those produced by anthracnose of eggplant (see Fact Sheet no. 390). Because of this, the lesions to examined under a microscope to determine if they are spores contaiing structures are those of Phomopsis (pycnidia) or (acervuli) type (Photos 6-8).
Look at seed under a lens, or binocular microscope, to detect round, black spore containing structures (pycnidia). Look for seeds that are discoloured, brownish or black. Alternatively, do a blotter test incubating seed on blotting paper for 7 days (12-hour light/dark), observing for pycnidia on the seed coat.
BIOSECURITY
Countries where Phomopsis vexans has not yet been introduced should consider all likely pathways for entry and apply quarantine measures accordingly. The risk of introduction of this fungus is high as it is seedborne and can also be spread on fruit.
BIOLOGICAL CONTROL
Trichoderma harzianum has been used as a treatment of nursery soil against Phomopsis vexans in trials in Bangladesh.
CULTURAL CONTROL
Before planting:
- Do not plant successive crops on the same land. Use a crop rotation of at least 3 years. Rotate, e.g., cucurbits, brassicas, onions, not with other members of the tomato/potato/chilli/capsicum family.
- Establish nurseries at distance from eggplant fields, not adjacent to them (Photo 9).
- Use commercial seed or treat seed with a fungicide before sowing. Alternatively, treat seed with hot water: 50oC for 25 minutes, then immediately cool seed in running water for 5 minutes, and dry. (Test on a small seed sample before treating the entire seed stock). Use a thermometer to measure temperature. Do not guess.
- Raise plants in nurseries using soilless potting mixes or pasteurise soil. Do not take soil from where eggplant has been grown previously.
- Remove plants from the nursery with leaf spots or wilt immediately they occur and burn.
During growth:
- Remove wilted plants and any infected fruit as they occur in the field and burn them.
- Avoid overhead irrigation in favour of drip irrigation. If not practical, then water early so that plants dry rapidly in the early morning sun.
- Encourage air movement to flow between plants by aligning rows in the direction of the wind. Space plants 60 cm in the row, and rows 90 cm apart.
After harvest:
- Collect and burn plants after final harvest, or plough in the plant debris deeply. Do not compost and use as mulch for succeeding crops; the fungus survives in crop debris.
RESISTANT VARIETIES
Breeding programs have sought resistance over many years, with moderate success. Check with seed companies for up-to-date information on tolerant/resistance varieties.
CHEMICAL CONTROL
Copper, chlorothanonil, mancozeb, or thiophanate-methyl for leaf and fruit infections. For seed treatments: hot water; and the following fungicides: captan, carbendazim, thiram, triadimenol, mancozeb, have all been used with effect. Triazole and strobilurin fungicides are likely to be effective, too.
____________________
When using a pesticide, always wear protective clothing and follow the instructions on the product label, such as dosage, timing of application, and pre-harvest interval. Recommendations will vary with the crop and system of cultivation. Expert advice on the most appropriate pesticide to use should always be sought from local agricultural authorities.
AUTHOR Grahame Jackson
Information from CABI (2010) Phomopsis vexans (Phomopsis blight of eggplant). Crop Protection Compendium. (https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.40488); and Eggplant insect pests & diseases. Home & Garden Information Center (2020) Fact Sheet HGIC 2224. Clemson University Cooperative Extension Service. (https://hgic.clemson.edu/factsheet/eggplant-insect-pests-diseases/); and Hot water treatment of seeds. UMass Extension Vegetable Program. University of Massachusetts Amherst. (https://ag.umass.edu/vegetable/news/hot-water-treatment-of-seeds) and from Nahar N, et al. (2019) Disease management in eggplant (Solanum melongena L.) nurseries also reduces wilt and fruit rot in subsequent plantings: A participatory testing in Bangladesh. Crop Protection 120: 113-124. (ttps://www.sciencedirect.com/science/article/pii/S026121941930064X?v(https://www.forestryimages.org/browse/detail.cfm?imgnum=5511752)ia%3Dihub). Photo 1 Brian Olson, Oklahoma State University, Bugwood.org. (https://www.forestryimages.org/browse/detail.cfm?imgnum=5511752). Photo 2 David B. Langston, University of Georgia, Bugwood.org. Photo 3 Brian Olson, Oklahoma State University, Bugwood.org.
Produced with support from the Australian Centre for International Agricultural Research under project HORT/2016/185: Responding to emerging pest and disease threats to horticulture in the Pacific islands, implemented by the University of Queensland and the Secretariat of the Pacific.