Brown rot of potato. It is also known as bacterial wilt of potato.
Pacific Pests, Pathogens, Weeds & Pesticides - Online edition
Pacific Pests, Pathogens, Weeds & Pesticides
Potato bacterial wilt Race 3 biovar 2 (479)
Ralstonia solanacearum Race 3 biovar 2 (R3bv2). Other names are: Bacillus solanacearum, Burkholderia solanacearum, and Pseudomonas solanacearum. There are five races of Ralstonia solanacearum: Race 1 has a host range of over 50 plant families (200 plant species), including chilli, capsicum, peanut, and Solanum species (but more rarely on potato); race 2 mostly affects banana and Heliconia; Race 3, geranium and potato; Race 4, ginger; and Race 5, mulberry.
An alternative system based on carbohydrate substrates divides strains into five biovars, with e.g., Race 1 corresponding to biovars 1, 3 and 4, and Race 3 to biovar 2, and Race 5 to biovar 5.
However, under a revised classification system (2005), and based on DNA sequencing, Ralstonia solanacearum has been divided into four groups, reflecting geography (phylotypes), and further by genetic sequence of an important gene (sequevars). By this method potato brown rot is Phylotype II, sequevars 1&2.
Asia, Africa, North, South and Central America, the Caribbean, Europe, Oceania. It is recorded from Australia, New Zealand, New Caledonia, and Papua New Guinea.
Potato, tomato, eggplant and other members of the Solanaceae; the ornamental geranium (Pelargonium hortorum) is also a host. There are many latent or symptomless weed hosts, e.g., Amaranthus species and Bidens pilosa (see Fact Sheet no. 467).
The bacterium is thought to have originated in the Andes of South America, and from there spread in potato tubers.
One of the most serious pathogens of potato, also tomato and other solanaceous crops. Infection occurs through wounds in the roots (e.g., caused by root-knot nematodes), or wherever young roots emerge.
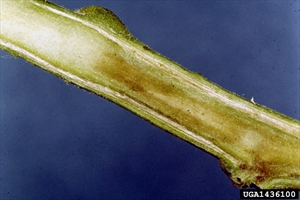
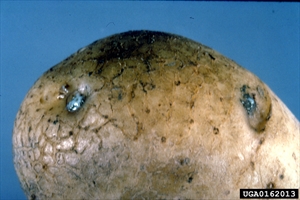
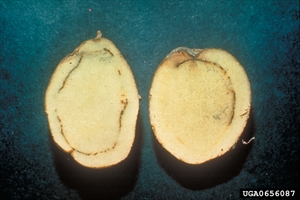
First signs are wilting of youngest leaves or leaflets during hot parts of the day with recovery at night (Photo 1). Later, plants may be yellowish, stunted, with leaves that droop, and stems that have brown narrow streaks. Internally, stems show browning of the vascular areas (Photo 2); a white bacterial ooze discharges from the cut ends best seen when placed in water (Photo 3). Ooze also forms at the buds or 'eyes' of tubers (Photo 4). Browning occurs in the vascular ring of the tuber as well (Photo 5). Eventually, plants collapse and die (Photo 6).
Although it occurs in countries where temperatures range from 24-35oC, it is best adapted for temperatures of about 27oC, i.e., lower (i.e., sub-tropical) compared to other races.
Spread to a limited extent occurs through wet soils over short distances, and in run-off water; over longer distances, spread is via infected tubers and in soil on machinery and shoes. There is the possibility of spread by insects. Survival in soil (and debris) is from 1-2 years depending on temperature, moisture, depth in the soil, presence of weeds (especially those of the Solanaceae) and volunteer potato plants. Survival on the outside of roots on non-hosts also occurs.
Annual losses due to the bacterium are estimated to be US1 billion each year, affecting some 3 m farm families over about 1.5 million hectares in some 80 countries, with particularly high losses reported from Greece, Israel, Nepal, and Venezuela. The eradication of an outbreak on geranium in greenhouses in the USA was put at US$10 million.
Look at cross sections of the stem to see brown discoloration of the vascular system. Look for bacterial streaming from stem pieces taken from a plant with symptoms and placed in a beaker of water. Look for ooze from slices of tubers; if not immediately evident, incubate tubers for 3-4 weeks at 30°C and observe for ooze from eyes. Confirmation of the exact genus, species and biovar requires examination by molecular methods using PCR.
BIOSECURITY
Countries that are still free from, but vulnerable to, brown rot of potato, need to: (i) define the risk; (ii) have preventive measures in place against an introduction; (iii) have quarantine protocols enacted in case a breach occurs; and (iv) be able to effect a rapid response against this bacterium in case eradication is a possibility, e.g., USDA New Pest Response Guidelines Ralstonia solanacearum race 3 biovar 2: (https://www.ippc.int/static/media/uploads/resources/new_pest_response_guidelines_ralstonia_solanacearum_race_3_biovar_2.pdf). If eradication fails, a recovery plan is required, such as that issued by the USDA, which includes disease management and research strategies, as well as education and extension priorities: (https://www.ars.usda.gov/ARSUserFiles/OPMP/NPDRS%20Recovery%20Plans/Recovery%20Plan%20for%20Ralstonia%20solanacearum%20R3b2_Final.pdf).
IPM STRATEGY
The control of brown rot of potato depends on an IPM strategy using several methods; the following is recommended.
CULTURAL CONTROL
Before planting:
- Use only certified 'seed' free from the potato brown rot for the last two growing seasons.
- Avoid cutting seed potato tubers because of the ease of spread of the bacterium during the process.
- Use bacterial wilt-resistant eggplant as rootstocks for wilt-susceptible tomatoes. Other (wild) Solanum species can also be used.
- Avoid planting crops in land where bacterial wilt has occurred previously; or if that is impossible, use a 2 to 3-year rotation. Brassicas, pulses, cereals, sweet potato and onion are considered to be effective rotations.
- If drainage is poor, plant on ridges or raised beds. And, if surrounding fields are prone to flooding, control floodwater flow to avoid contaminating potato fields.
During growth:
- Remove wilted plants as soon as they are seen, taking as much soil from around the roots as possible, and burn the plants and soil. Do not let infested soil fall on neighbouring healthy plants.
- Remove soil from machinery, tools and shoes and wash with water after working in brown rot contaminated fields, especially before traveling to fields free from the bacterium.
- For tomatoes and capsicum grown in small numbers, use containers with pasteurised soil if fields are infested with the bacterium.
After harvest:
- Use a crop rotation of at least 2 years (preferably 4 years), and during that time avoid susceptible crops (and remove weeds) in the Solanaceous family. Note, crop rotation is only of limited use against this disease because of long survival in the soil, and its wide host range. Rotations with maize, soybeans, grasses and rice have been used in rotations elsewhere.
- Collect debris and dispose by burning.
RESISTANT VARIETIES
Some potato varieties are less susceptible, but none are resistant. Breeding continues. There are capsicum, eggplant and tomatoes lines with varying degrees of resistance. Check lines available from AVRDC, The World Vegetable Center, Taiwan.
CHEMICAL CONTROL
There are no chemicals (except soil fumigants) that can be used to give satisfactory control of bacterial wilt.
AUTHOR Grahame Jackson
Information from Charkowski A, et al. (2020) Bacterial Diseases of Potato. In: Campos H, Ortiz O (eds) The Potato Crop. Springer, Cham. (https://doi.org/10.1007/978-3-030-28683-5_10); and Lemay A, et al. (2003) Pest Data Sheet Ralstonia solanacearum race 3 biovar 2. USDA/APHIS/PPQ Center for Plant Health Science and Technology Plant Epidemiology and Risk Analysis Laboratory Raleigh, NC. USA. (https://plantpath.ifas.ufl.edu/rsol/RalstoniaPublications_PDF/USDARalstoniaPestDataSheet_2003.pdf); and from Sullivan M, et al., (2013) CPHST Pest Datasheet for Ralstonia solanacearum race 3 biovar 2. USDA-APHIS-PPQ-CPHST. (http://download.ceris.purdue.edu/file/1610). Photo 1 Anare Caucau, Koronivia Research Station, MoA, Fiji. Photo 2 Clemson University - USDA Cooperative Extension Slide Series, Bugwood.org. Photo 4 Central Science Laboratory, Harpenden , British Crown, Bugwood.org. Photo 5 Ministry of Agriculture and Rural Affairs, Bugwood.org (https://www.forestryimages.org/browse/subthumb.cfm?sub=10469). Photo 6 National Plant Protection Organization, the Netherlands.
Produced with support from the Australian Centre for International Agricultural Research under project HORT/2016/185: Responding to emerging pest and disease threats to horticulture in the Pacific islands, implemented by the University of Queensland and the Secretariat of the Pacific Community.