This article complements the introductory essay about eucalypts included in the "Learn about Eucalypts" section. Its aim is to provide an up-to-date account of the outcomes of research derived from different groups during the past 5 years relating to relationships within Eucalyptus s.s. As such it includes only those publications and hypotheses relating to higher level relationships of major groupings within the eucalypts. Some of the research reported below also provides insights into biogeographic relationships of the eucalypt group – in large part these are not the focus of this article and are not discussed in detail.
Introduction
The first comprehensive classification of the eucalypts was published by Blakely in 1934, in which he treated more than 600 taxa, building on earlier work of Maiden and Mueller. Blakely's classification remained the critical reference for Eucalyptus taxonomists for the next 37 years when a new but informal classification was published by Pryor and Johnson (1971). In this work the authors divided the genus into seven subgenera, and although of an informal nature, presented a system of great advance on Blakely's treatment. The small genus Angophora was retained.
The next 20 years saw much debate about the naturalness of Eucalyptus and whether other genera should be recognized (e.g., Johnson 1987). Based on morphological data, Hill and Johnson in 1995 proposed a split in the genus and recognition of the genus Corymbia. This new genus of c. 113 species, comprised the ghost gums and the bloodwoods, and Hill and Johnson concluded that Corymbia is the sister group to Angophora, with the synapomorphy of the distinctive cap cells on bristle glands (Ladiges 1984) being unambiguous. The remaining taxa in Eucalyptus were not treated by Hill and Johnson in a formal taxonomic sense.
In 2000, Brooker published a formal classification of the eucalypts, which was a synthesis in the form of an updated taxonomy to accommodate the numerous taxa published since Chippendale's 1988 treatment. This work recognized one genus, Eucalyptus and included Angophora and Corymbia as two of a total of 13 subgenera. The treatment assigned all species accepted by Brooker and published prior to 1999 to a hierarchical system of subgenera, sections, subsections, series, subseries and supraspecies (Brooker 2000). The approx. 800 species of Eucalyptus were divided into 13 subgenera, two of which were the ghost gums (subgenus Blakella) and bloodwoods (subgenus Corymbia), which constitute Hill and Johnson's single genus Corymbia.
This new classification of the eucalypts by Brooker (2000) based on comparative morphology, stimulated further debate as to whether Angophora and Corymbia should be included within Eucalyptus s.s., and also whether the bloodwood group Corymbia is monophyletic.
In the second edition of EUCLID (Brooker et al. 2002), while considerable discussion was still running in the botanical community about the acceptance of Corymbia as a monophyletic group, a conservative approach was adopted in which both genera Eucalyptus and Angophora were recognized in a traditional sense.
The eucalypt group
To place the discussion of relationships across the eucalypts in context it is worth reiterating here the seven genera for which there is general agreement of what constitutes 'the eucalypt group'. Eucalyptus L'Hér. is the largest genus with more than 660 species occurring primarily in Australia, and with a few species in Indonesia, the Philippines, Timor and New Guinea. The sister group to Eucalyptus is the lineage containing Corymbia Hill & Johnson (c. 90 species) widespread in northern Australia, extending to New Guinea, and Angophora Cav. (9 taxa) restricted to eastern Australia.
Four other smaller genera make up the eucalypt group. Arillastrum Pancher & Baill. is a monotypic genus (A. gummiferum) endemic to New Caledonia; Allosyncarpia S.T.Blake from Arnhem Land in northern Australia is also monotypic (A. ternata); a third monotypic genus Stockwellia D.J.Carr, S.G.M.Carr & B.Hyland is restricted to the Atherton Tableland of north Queensland (S. quadrifida). The genus Eucalyptopsis C.T.White comprises two species, E. papuana and E. alauda and is found in New Guinea and the Moluccan Archipelago.
Phylogenetic hypotheses
Despite the considerable debate surrounding the classification of the eucalypts based on differing interpretations of morphological features and their significance in evolutionary history, cladistic analyses of morphological and molecular datasets show a high degree of agreement on the higher level phylogeny of the group. Summaries and accounts of major pieces of work contributing to our understanding of eucalypt relationships follow.
Udovicic & Ladiges (2000) used a number of different nuclear and chloroplast DNA regions to examine their informativeness in understanding the phylogeny of the genera in the eucalypt group. Parsimony analyses of this sequence data from all the DNA regions revealed three major clades in all cases – the basal Stockwellia (the undescribed "Myrtaceae sp." at that time), Eucalyptopsis and Allosyncarpia clade, the Angophora and Corymbia clade and the Eucalyptus clade. The position of the seventh genus of the eucalypt group, Arillastrum, was not well resolved and showed different relationships to the other clades depending on the DNA region analysed. The combined (total evidence) dataset (Figure 1- from Udovicic & Ladiges 2000) depicts a resolved relationship of Arillastrum as sister to the Angophora-Corymbia and Eucalyptus clades, but these basal nodes were not well supported. The authors concluded that there was not enough sequence variation present to resolve the exact position of Arillastrum.
All of the analyses in Udovicic & Ladiges (2000) indicated Angophora and Corymbia to be a monophyletic group separate from Eucalyptus. These relationships were congruent with those of earlier studies based on morphology and molecular data (Hill & Johnson 1995; Ladiges et al. 1995; Udovicic et al. 1995; Steane et al. 1999).
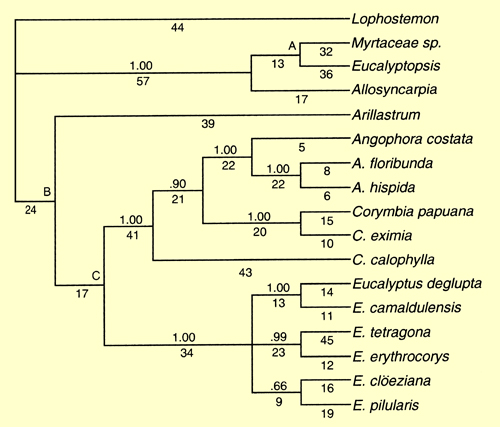
Figure 1. Strict consensus tree, based on a combined data set for 16 ingroup taxa and the outgroup, Lophostemon confertus. Including 13 indels, there were 313 informative characters yielding three equally parsimonious trees of length 693, CI = 0.60, RI = 0.69. Jackknife values greater than 0.50 are shown above branches and letters A, B & C denote nodes with a jackknife value less than 0.50 that could be collapsed. Numbers of characters supporting a node are shown below branches. (Source: Udovicic & Ladiges 2000, Fig.5)
Steane et al. (2002) undertook an expanded survey, following their earlier study (Steane et al. 1999), using ITS sequences for 90 species of Eucalyptus s.s. and 28 species of related eucalypt genera and outgroups. Their study revealed phylogenetic information for higher level relationships among the eucalypts and also between sections and subgenera.
Their results indicated that Angophora and Corymbia form a well supported clade differentiated from Eucalyptus s.s. and they argued that the ITS results provided evidence that the two genera should not be regarded as subgenera of Eucalyptus without clear indication of the relationships between Eucalyptus s.s. and the other eucalypt genera. They suggested that if the genus Eucalyptus is to be expanded to include Angophora and Corymbia (sensu Brooker 2000), the ITS data implied that Allosyncarpia, Eucalyptopsis and Stockwellia, and potentially Arillastrum should also be included in Eucalyptus.
Their study showed Allosyncarpia, Eucalyptopsis, Stockwellia and Arillastrum were also separated from Eucalyptus (Figure 2 – from Steane et al. 2002). The long branch lengths in this analysis, however, point to the divergent nature of the relationships distinguishing Angophora + Corymbia, Allosyncarpia, Eucalyptopsis, Stockwellia and Arillastrum from each other and from Eucalyptus s.s.
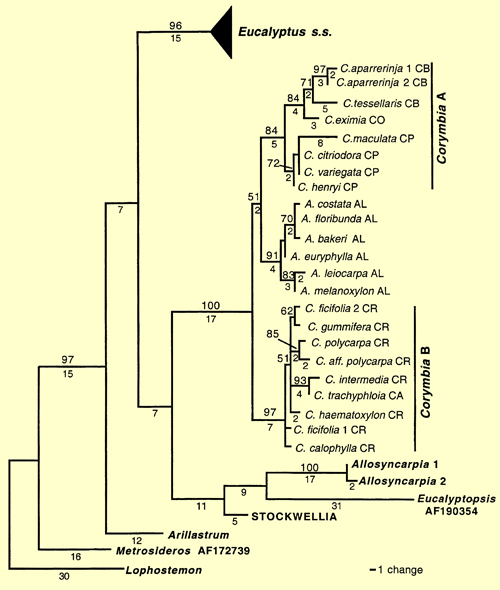
Figure 2. Phylogram of one of the 18695 FDS cladograms, detailing relative positions of Allosyncarpia, Arillastrum, Angophora, Corymbia, Eucalyptopsisand 'Stockwellia' relative to Eucalyptus s.s., when rooted on Lophostemon (see text). Clades representing 'Corymbia A' (Clade A) and 'Corymbia B' (Clade B) are indicated. Branch lengths are shown below branches; numbers are not shown when branch length = 1. Bootstrap percentages greater than 50% are shown above branches. Letters following species names represent taxon codes (Table 1). The triangle leading to Eucalyptus s.s. represents multiple terminal taxa within that clade. STOCKWELLIA–two samples of 'Stockwellia' had identical ITS sequences and were reduced to a single operational taxonomic unit. Tree topology, branch lengths and bootstrap support from the AFOG analysis are very similar to those in this figure. (Source: Steane et al. 2002, Fig.2)
In contrast, the branch lengths within Eucalyptus itself are relatively short and the authors support a case for maintaining Angophora + Corymbia, Allosyncarpia, Eucalyptopsis, Stockwellia, Arillastrum and Eucalyptus as separate genera.
The analysis of the full data set places Arillastrum near the base of the cladogram, as sister to all other eucalypts.
Increased sampling using ITS data did not resolve the relationship between Angophora and Corymbia, but showed Corymbia to be paraphyletic with Angophora nested within. Here Angophora is sister to Corymbia 'A' the yellow bloodwoods (informal sect. Ochraria), the paper-fruited bloodwoods or ghost gums (informal sect. Blakearia) and the spotted gums (informal sect. Politaria). This is congruent with results of earlier studies based on cpDNA data (Udovicic and Ladiges 2000).
Whittock et al. (2003) mainly relates to the tropical box species (subgenus Minutifructus), but chloroplast data provide further evidence for the genetic differentiation of Angophora and Corymbia (together with Arillastrum) from Eucalyptus. It also showed Corymbia to be paraphyletic. This study indicated that the chloroplast DNA data may be potentially informative in revealing relationships within the eucalypts, and will be better tested with more complete taxon sampling.
Ladiges et al. (2003) used the current state of phylogenetic analysis of the eucalypts (and the 'melaleuca group'), together with geological events and fossil evidence to explore further Australian biogeographical patterns and to test historical connections of biotas.
These authors presented the current knowledge of phylogenetic relationships of the seven genera (Figure 3 – from Ladiges et al. 2003) based on both nuclear and chloroplast DNA as reported by Udovicic & Ladiges (2000). This strict consensus tree revealed two major lineages within the eucalypt group, corresponding to earlier recognised Eucalyptopsis and Eucalyptus alliances of Briggs & Johnson (1979).
The position of Arillastrum and its relationship with the Angophora-Corymbia and Eucalyptus groups led Ladiges et al. (2003) to propose that the divergence of Arillastrum from Angophora, Corymbia and Eucalyptus corresponded to the geological vicariance event when the Lord Howe Rise and Norfolk Ridge (including New Caledonia) detached from the Australia-Antarctic land mass. They concluded that this split of ancestral taxa began in the late Cretaceous, about 65-70 million years ago. In applying molecular dating techniques to a study of southern hemisphere Myrtales Sytsma et al. (2004) also showed the eucalypt lineage to date back to the late Cretaceous.
Ladiges et al. (2003) discuss the differentiation of clades and phylogenetic patterns in relation to major geological and climatic events.
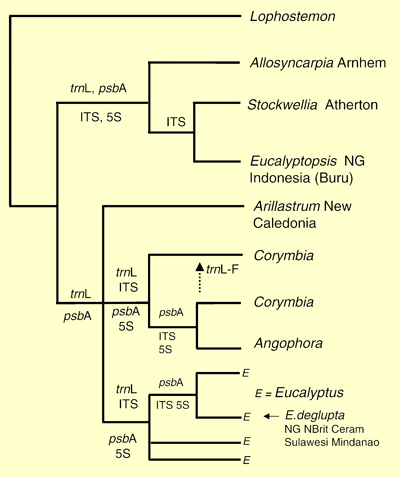
Figure 3. Summary molecular phylogeny of the eucalypt group based on both nuclear (ITS and 5 S rDNA spacer regions) and chloroplast DNA sequence data (psbA–trnH intergenic spacer, trnL intron and trnL–F spacer regions; Lophostemon is an outgroup taxon. The data sets that support particular nodes are shown (based on Udovicic & Ladiges, 2000, strict consensus tree, Fig. 5, p. 643, Fig. 1 this document) (Source: Ladiges et al. 2003, Fig. 2)
Crisp et al. (2004) included the eucalypts among other groups of community-dominant Australian plants, such as Casuarinaceae, pea-flowered legumes and Banksia, in their analysis combining fossil evidence with molecular phylogenies to provide insights on the history of radiation and extinction of the flora of Australia.
The study used the approach of molecular dating to allocate dates to nodes of a eucalypt phylogenetic tree based on ITS sequence data from Steane et al. (2002). The eucalypt chronogram (Crisp et al. 2004, Fig. 3) was calibrated using the assumption of Ladiges et al. (2003) that a vicariance event c. 70 million years ago isolated Arillastrum in New Caledonia from the rest of the eucalypts in Australia.
The molecular dating approach does throw some insight on the radiation of the group within Australia. Using the calibration of 70 million years ago for the divergence of the eucalypts from Arillastrum, Crisp et al.'s molecular dating suggests the diversification of eucalypts proceeded steadily for at least 30 million years before Australia became isolated from Antarctica, and continued through the mid-Cenozoic.
While Crisp et al. (2004) do not provide any new evidence contributing to the understanding of the relationships within the eucalypts, the chronogram (their Fig. 3) that they present (one of several equally parsimonious solutions) reflects some slightly different topology from previous analyses. For instance, the positions of Arillastrum and that of the Corymbia + Angophora clade is different from other cladograms based on chloroplast DNA (Whittock et al. 2003), and molecules and morphology (Udovicic and Ladiges 2000; Ladiges et al. 2003).
In raising concerns about deriving dates from sequence data, Ladiges and Udovicic (2005) make comparisons between some of the hypotheses proposed by Crisp et al. (2004) and those of Ladiges et al. (2003). In particular they discuss the conclusions drawn from molecular dating and biogeographic approaches with respect to the Eucalyptopsis-Allosyncarpia-Stockwellia clade and relationships between these three genera.
In the most recent work on the eucalypts, Parra-O et al. (2006) sequenced the ETS region of nuclear ribosomal DNA for an extensive range of taxa to specifically address the question of monophyly of Corymbia and to clarify relationships within the eucalypt group. The ETS dataset was combined with ITS sequences from previous studies of Steane et al. (2002) and Udovicic and Ladiges (2000).
The analyses of the combined ETS and ITS sequences resulted in three well supported clades – the Corymbia + Angophora clade, Eucalyptus and the Eucalyptopsis group (Figure 4 – from Parra-O et al. 2006). Corymbia is shown to be monophyletic, and Eucalyptus is sister to the combined Corymbia + Angophora clade. This research provides evidence for the recognition of Corymbia and Angophora as separate genera.
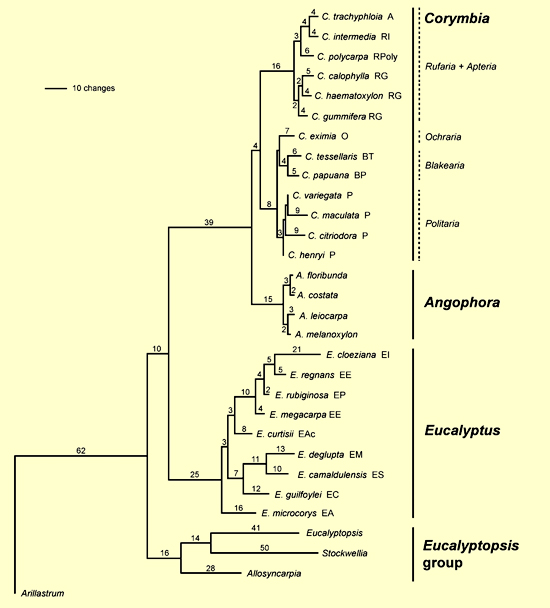
Figure 4. One of the six most parsimonious trees obtained from analysis of the combined dataset (ETS + ITS), showing branch lengths. Letters following species and group names represent taxon codes. Corymbia: A = Sect. Apteria; B = Sect. Blakearia(BP, ser. Papuanae; BT, ser. Tessellares); O = Sect. Ochraria; P = Sect. Politaria; R = Sect. Rufaria (RG, ser. Gummiferae; RI, ser. Intermediae; RPoly, ser. Polycarpae). Eucalyptus:EA, subg. Alveolata; Eac, subg. Acerosa;EC,subg. Cruciformes; EE,subg. Eucalyptus; EI, subg. Idiogenes;EM,subg. Minutifructus;EP,subg. Primitiva; ES,subg. Symphyomyrtus. (Source: Parra-O et al. 2006, Fig. 4)
There is strong support for the Corymbia + Angophora clade and the authors emphasise that this grouping is recovered in all molecular phylogenetic studies to date, including the use of two other nuclear and seven chloroplast regions (as discussed above). The ETS and ITS analyses again reveal the long branch to the Corymbia + Angophora clade with the implication that this is a deep divergence from Eucalyptus.
This combined analysis of ETS and ITS does not resolve unequivocally the relationships at the basal nodes of the eucalypts. The Eucalyptopsis group is shown to be outside the Corymbia + Angophora and Eucalyptus clade.
The analyses of Parra-O et al. (2006) reveal the monophyly of the Eucalyptopsis group, consistent with the results of earlier molecular studies, and supported by morphological characters (Bohte and Drinnan 2005). Within the Eucalyptopsis group there is strong support for Allosyncarpia as sister to the clade of Stockwellia + Eucalyptopsis. The latter sister relationship has been identified in some molecular analyses and is supported with morphology data, with Stockwelliaand Eucalyptopsis sharing undifferentiated perianth parts and elongated fusiform buds as discussed by Bohte and Drinnan (2005).
Summary
It is likely that the current level of activities surrounding the eucalypts will result in further hypotheses being proposed, and potentially, additional changes in eucalypt classification. One implication of this increased knowledge is that some researchers may recommend additional changes in eucalypt nomenclature.
The taxonomic and nomenclatural situation in Eucalyptus sens lat. is common to many large economically and environmentally important plant groups, i.e., there is a dynamic tension between presenting a natural classification reflecting phylogenetic relationships and the pragmatic treatment that maintains nomenclatural stability.
We have adopted here in EUCLID - Eucalypts of Australia some of the recent proposed changes, namely recognising the current wide acceptance of Angophora and Corymbia as genera distinct from Eucalyptus. Over time and with new information and different interpretations it is possible that the prevailing consensus may shift in different directions. We note that there is no universally accepted circumscription for these icons of the Australian flora, but, to the best of our ability we believe that EUCLID reflects the most widely accepted nomenclatural concepts.
Judy West
Complete Reference List
References (for this synopsis)
Blakely, W.F. (1934). " A Key to the Eucalypts", 1st edn. The Worker Trustees, St. Andrew's Place, Sydney.
Bohte, A. and Drinnan, A. (2005). Floral development and systematic position of Arillastrum, Allosyncarpia, Stockwellia and Eucalyptopsis (Mrytaceae). Pl. Syst. Evol. 251: 53-70.
Briggs, B.G. and Johnson, L.A.S. (1979). Evolution in the Myrtaceae – evidence from inflorescence structure. Proc. Linnean Society of New South Wales 102: 157-256.
Brooker, M.I.H. (2000). A new classification of the genus Eucalyptus L'Hér. (Myrtaceae). Australian Systematic Botany 13: 79-148.
Brooker, M.I.H, Slee, A.V., Connors, J.R. and Duffy, S.M. (2002). EUCLID – Eucalypts of southern Australia. CD. CSIRO Publishing, Melbourne.
Crisp, M.D., Cook, L.G. and Steane, D.A. (2004). Radiation of the Australian flora: what can comparisons of molecular phylogenies across multiple taxa tell us about the evolution of diversity in present-day communities? Phil. Trans. R. Soc. Lond. B 359: 1551-1571.
Crisp, M. D., Cook, L. G., and Steane, D. A. (2005). Molecular dating and eucalypts: reply to Ladiges and Udovicic. Australian Systematic Botany 18: 295-296.
Hill, K.D. and Johnson, L.A.S. (1995). Systematic studies in the Eucalypts 7. A revision of the bloodwoods, genus Corymbia (Myrtaceae). Telopea 6: 185-504.
Johnson, L.A.S. (1987). Aspects of the systematics of the eucalypts. Australian Systematic Botany Society Newsletter 53: 91-93.
Ladiges, P.Y. (1984). A comparative study of trichomes in Angophora Cav. and Eucalyptus L'Hér. – a question of homology. Austral. J. Bot. 32: 561-574.
Ladiges, P.Y. and Udovicic, F. (2005). Comment on molecular dating of the age of eucalypts. Australian Systematic Botany 18: 291-293.
Ladiges, P.Y., Udovicic, F. and Drinnan, A.N. (1995). Eucalypt phylogeny – molecules and morphology. Australian Systematic Botany 8: 483-487.
Ladiges, P.Y., Udovicic, F. and Nelson, G. (2003). Australian biogeographical connections and the phylogeny of large genera in the plant family Myrtaceae. Journal of Biogeography 30: 989-998.
Parra-O, C., Bayly, M., Udovicic, F. and Ladiges, P. (2006). ETS sequences support the monophyly of the eucalypt genus Corymbia (Myrtaceae). Taxon 55: 653-663.
Pryor, L.D. and Johnson, L.A.S. (1971). A classification of the eucalypts. Australian National University Press, Canberra.
Steane, D.A., McKinnon, G.E., Vaillancourt, R.E. and Potts, B M. (1999). ITS sequence data resolve higher level relationships among the eucalypts. Molecular Phylogenetics and Evolution 12: 215-223.
Steane, D.A., Nicolle, D., McKinnon, G.E., Vaillancourt, R.E. and Potts, B M. (2002). Higher level relationships among the eucalypts are resolved by ITS-sequence data. Australian Systematic Botany 15: 49-62.
Sytsma, K.J., Litt, A., Zjhra, M.L., Pires, C., Nepokroeff, M., Conti, E., Walker, J. and Wilson, P.G. (2004). Clades, clocks, and continents: historical and biogeographical analysis of Myrtaceae, Vochysiaceae, and relatives in the shouthern hemisphere. International Journal of Plant Sciences 165 (4 Suppl): S85-S105.
Udovicic, F. and Ladiges, P.Y. (2000). Informativeness of nuclear and chloroplast DNA regions and the phylogeny of the eucalypts and related genera (Myrtaceae). Kew Bulletin 55: 633-645.
Udovicic, F., McFadden, G.I. and Ladiges, P.Y. (1995). Phylogeny of Eucalyptus and Angophora based on 5S rDNA spacer sequence data. Molecular Phylogenetics and Evolution 4: 247-256.
Whittock, S., Steane, D.A., Vaillancourt, R.E. and Potts, B.M. (2003). Molecular evidence shows that the tropical boxes (Eucalyptus subgenus Minutifructus) are over-ranked. Transactions of the Royal Society of South Australia 127: 27-32.





